同期放化疗后的巩固化疗可以使局部晚期非小细胞肺癌患者获益吗?
2012-05-28 11:56:14 来源: 丁香园 作者: 评论:0 点击:
丁香园 作者: 评论:0 点击:
Is consolidation chemotherapy after concurrent chemoradiotherapy beneficial for locally advanced non-small cell lung cancer? A pooled analysis of the literature.
同期放化疗后的巩固化疗可以使局部晚期非小细胞肺癌患者获益吗?一项相关文献的荟萃分析
Background: There is a growing interest on the efficacy of maintenance chemotherapy to metastatic non-small cell lung cancer (NSCLC) and adjuvant chemotherapy to early stage NSCLC. However, the role of consolidation chemotherapy (CCT) after concurrent chemo-radiotherapy in locally advanced NSCLC is undetermined.
背景:人们越来越多的关注于转移性非小细胞肺癌的维持化疗和早期非小细胞肺癌的辅助化疗的效果。然而,局部晚期非小细胞肺癌同期放化疗后的巩固化疗的作用仍未确定。
Methods: We systematically searched PubMed for phase II/III trials examining survival of locally-advanced NSCLC treated with concurrent chemo-radiotherapy between January 1, 1995 and October 31, 2011.
方法:我们在PubMed上系统检索了从1995年1月1日至2011年11月31日之间的相关II/III临床试验,这些临床试验都是评价接受同期放化疗治疗的局部晚期非小细胞肺癌患者生存的研究。
Median overall survival (mOS) and corresponding 95% confidence interval (CI) were collected from each study and pooled.
收集每个研究中相关数据,包括:中位生存期和相应的95%可信区间,并进行汇总。
We extracted log-transformated hazards and its standard errors under the assumption that survival follows an exponential distribution, and computed a pooled mOS and its 95% CI using random-effect model.
在假设生存期服从指数分布的情况下,我们提取指数转换风险及其标准误,并应用随机效果模型计算总的中位生存期和95%可信区间。
Collected trial arms were divided into two groups by the presence of CCT: Arm with CCT (CCT+) and without CCT (CCT-).
纳入的临床试验各组数据根据是否接受巩固化疗分为两组:接受巩固化疗组和未接受巩固化疗组。
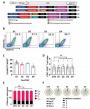
Results: Forty-five studies were identified including 9 phase III studies and 36 phase II studies with 51 arms (CCT+: 29, CCT-: 22).
结果:45个研究被纳入,其中包括9个III期临床试验和36个II期临床试验,共51组(接受巩固化疗组29例,未接受巩固化疗组22例)
Clinical data were comparable in clinical stage, performance status, cancer histology, gender, and median age between the two groups.
两组间临床分期,体力状态,组织学,性别,以及中位年龄等临床数据具有可比性。
I2 values for assessing heterogeneity were 15.3, 9.1 and 24.2% in overall, CCT+ and CCT- studies, respectively.
评价异质性的I2值分别为总体15.3%, 巩固化疗组9.1% and 未行巩固化疗组24.2%。
There was no statistical difference in pooled mOS between CCT+ (18.5 month, 95%CI: 16.7-20.5) vs CCT- (18.1 month, 95%CI: 16.5-20.2).
两组间总的生存期无统计学差异,巩固化疗组(18.5 month, 95%CI: 16.7-20.5),未行巩固化疗组(18.1 month, 95%CI: 16.5-20.2)
In regard to the ≥ grade 3 toxicities in pneumonitis, esophagitis, and neutropenia, there was no difference between the two groups throughout the whole treatment courses.
在整个治疗过程中,两组间3级以上的毒性反应,包括肺炎,食管炎,中性粒细胞减少均无差异。
In random effect models, predicted hazard ratio of CCT+ to CCT- was 0.98 (95%CI: 0.8-1.13, p=0.7574).
关于疗效模型,巩固化疗组与未行巩固化疗组的预测风险比为0.98 (95%CI: 0.8-1.13, p=0.7574).
These models estimated that addition of CCT could not yield more than 3 months of survival prolongation for patients with locally advanced NCSLC.
依据这些模型估计,对于局部晚期非小细胞肺癌患者,同期放化疗后加用巩固化疗并不能产生3个月以上的生存获益。
Conclusions: The pooled analysis on publication basis failed to provide evidence that consolidation chemotherapy yields significant survival benefit for locally advanced NSCLC
结论:我们这项基于已出版文献的荟萃分析并没有发现同期放化疗后巩固化疗使局部晚期非小细胞肺癌患者产生明显的生存获益。
同期放化疗后的巩固化疗可以使局部晚期非小细胞肺癌患者获益吗?一项相关文献的荟萃分析
Background: There is a growing interest on the efficacy of maintenance chemotherapy to metastatic non-small cell lung cancer (NSCLC) and adjuvant chemotherapy to early stage NSCLC. However, the role of consolidation chemotherapy (CCT) after concurrent chemo-radiotherapy in locally advanced NSCLC is undetermined.
背景:人们越来越多的关注于转移性非小细胞肺癌的维持化疗和早期非小细胞肺癌的辅助化疗的效果。然而,局部晚期非小细胞肺癌同期放化疗后的巩固化疗的作用仍未确定。
Methods: We systematically searched PubMed for phase II/III trials examining survival of locally-advanced NSCLC treated with concurrent chemo-radiotherapy between January 1, 1995 and October 31, 2011.
方法:我们在PubMed上系统检索了从1995年1月1日至2011年11月31日之间的相关II/III临床试验,这些临床试验都是评价接受同期放化疗治疗的局部晚期非小细胞肺癌患者生存的研究。
Median overall survival (mOS) and corresponding 95% confidence interval (CI) were collected from each study and pooled.
收集每个研究中相关数据,包括:中位生存期和相应的95%可信区间,并进行汇总。
We extracted log-transformated hazards and its standard errors under the assumption that survival follows an exponential distribution, and computed a pooled mOS and its 95% CI using random-effect model.
在假设生存期服从指数分布的情况下,我们提取指数转换风险及其标准误,并应用随机效果模型计算总的中位生存期和95%可信区间。
Collected trial arms were divided into two groups by the presence of CCT: Arm with CCT (CCT+) and without CCT (CCT-).
纳入的临床试验各组数据根据是否接受巩固化疗分为两组:接受巩固化疗组和未接受巩固化疗组。
Results: Forty-five studies were identified including 9 phase III studies and 36 phase II studies with 51 arms (CCT+: 29, CCT-: 22).
结果:45个研究被纳入,其中包括9个III期临床试验和36个II期临床试验,共51组(接受巩固化疗组29例,未接受巩固化疗组22例)
Clinical data were comparable in clinical stage, performance status, cancer histology, gender, and median age between the two groups.
两组间临床分期,体力状态,组织学,性别,以及中位年龄等临床数据具有可比性。
I2 values for assessing heterogeneity were 15.3, 9.1 and 24.2% in overall, CCT+ and CCT- studies, respectively.
评价异质性的I2值分别为总体15.3%, 巩固化疗组9.1% and 未行巩固化疗组24.2%。
There was no statistical difference in pooled mOS between CCT+ (18.5 month, 95%CI: 16.7-20.5) vs CCT- (18.1 month, 95%CI: 16.5-20.2).
两组间总的生存期无统计学差异,巩固化疗组(18.5 month, 95%CI: 16.7-20.5),未行巩固化疗组(18.1 month, 95%CI: 16.5-20.2)
In regard to the ≥ grade 3 toxicities in pneumonitis, esophagitis, and neutropenia, there was no difference between the two groups throughout the whole treatment courses.
在整个治疗过程中,两组间3级以上的毒性反应,包括肺炎,食管炎,中性粒细胞减少均无差异。
In random effect models, predicted hazard ratio of CCT+ to CCT- was 0.98 (95%CI: 0.8-1.13, p=0.7574).
关于疗效模型,巩固化疗组与未行巩固化疗组的预测风险比为0.98 (95%CI: 0.8-1.13, p=0.7574).
These models estimated that addition of CCT could not yield more than 3 months of survival prolongation for patients with locally advanced NCSLC.
依据这些模型估计,对于局部晚期非小细胞肺癌患者,同期放化疗后加用巩固化疗并不能产生3个月以上的生存获益。
Conclusions: The pooled analysis on publication basis failed to provide evidence that consolidation chemotherapy yields significant survival benefit for locally advanced NSCLC
结论:我们这项基于已出版文献的荟萃分析并没有发现同期放化疗后巩固化疗使局部晚期非小细胞肺癌患者产生明显的生存获益。
上一篇:可切除的结直肠癌肝转移治疗之我见
下一篇:BRAF 靶向癌症治疗
频道总排行
频道本月排行
热门购物
评论排行
- 2011年临床执业医师考试实践技能真...(13)
- 腋臭手术视频(12)
- 2008年考研英语真题及参考答案(5)
- 节食挑食最伤女人的免疫系统(5)
- 核辐射的定义和单位(5)
- CKD患者Tm与IMT相关(5)
- 齐鲁医院普外科开展“喉返神经监护...(5)
- windows7激活工具WIN7 Activation v1.7(5)
- 正常微循环(5)
- 美大学性教育课来真的 男女上阵亲...(4)