美敦力公司股票因其药物涂布支架的问题而滑落!
2009-05-26 20:36:34 来源: 作者: 评论:0 点击:
Shares of the medical device maker Medtronic fell Thursday after a study showed that its new drug-coated stent was associated with more heart attacks and blood clots than a rival stent made by Johnson & Johnson.
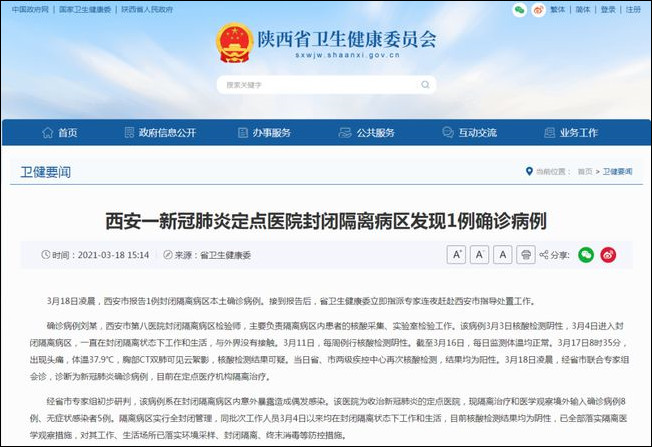
星期四一项研究结果显示医疗器械公司Medtronic的药物涂布支架与其竞争对手Johnson & Johnson公司同类产品相比具有更高的心脏事件和血栓发生率。其公司股票价格应声下降。
Results from the study, which included more than 2,000 patients, showed that heart patients who received the Medtronic device, called Endeavor, had more heart attacks and blood clots and needed repeat procedures more often than those treated with the Johnson & Johnson stent, known as Cypher.
这项纳入2000多名患者的研究结果显示:植入Medtronic公司的支架- Endeavor-的心脏病患者其心脏事件和血栓发生率以及重复接受手术率要远高于这些应用Johnson & Johnson公司的Cypher的患者。
There was no difference in death rates, however, according to the study, called Sort Out III — which was sponsored by Johnson & Johnson and presented at the Transcatheter Cardiovascular Therapeutics meeting in Washington.
然而,根据这项由Johnson & Johnson公司发起并在华盛顿心血管腔内治疗会议上发布的名为Sort Out III的研究结果显示应用两种产品的死亡率无明显差异。
A Medtronic spokesman, Joe McGrath, said the study was at odds with every other study that has been done.
Medtronic的发言人- Joe McGrath –宣称这项研究与其他各项研究结果不符。
“The data are also short-term,” he said in a statement. “At nine months, it’s premature to draw any meaningful conclusions about a difference between Cypher and Endeavor.”
“这项研究的数据是来自于短期研究”他在一项声明中说“在9个月时间内,希望得出任何关于Cypher和 Endeavor之间差别都是不成熟的。”
“Sort Out III at nine months has no bearing on what matters most to patients with coronary artery disease — long-term freedom from adverse events and long-term freedom from repeat procedures,” he added.
他又说到“Sort Out III在9个月时间内,并没有对冠状动脉及病患者所要达到的重要目标进行研究—长期无副作用和长期免于重复手术”。
In a statement, Dr. Campbell Rogers, chief scientific officer at Johnson & Johnson’s cardiovascular unit, the Cordis Corporation, said, “The breadth of clinical safety and efficacy data and the long-term patient outcomes associated with the Cypher stent are completely unmatched by the competition, and this gap continues to widen.”
Johnson & Johnson 公司心血管部- the Cordis Corporation -的首席科学家Dr. Campbell Rogers在一项声明中说道“Cypher支架与其他支架相比其临床安全性和有效性数据以及患者的长期预后存在差距,而这种差距仍在加大”。
A Leerink Swann analyst, Rick Wise, said he thought the market had overreacted to the data.
Leerink Swann的一位分析员Rick Wise说道他认为市场对这项数据的反应是过激的。
He noted that the study did not directly compare the two devices and that the registries raised questions about, among other things, patient complexity and optimal use of anticlotting drugs, both of which are crucial variables in driving adverse events.
他宣称这项研究并没有对这两种支架直接进行比较。研究者在许多事物中对患者的复杂性和选用适当的涂布药物提出疑问,而这两项是避免副作用的重要参数。
David Lewis, an analyst with Morgan Stanley, characterized the results as a “modest negative” for Medtronic, which already is struggling to defend its market share.
Morgan Stanley的一名分析员David LewisMedtronic认为这项结果对Medtronic来说是一种“适中的负面效应”,而该公司正为维持其股票而挣扎。
Drug-coated stents are tiny wire-mesh tubular devices that are implanted in diseased vessels to prop them open. The drug coating aims at keeping vessels from reclogging.
药物涂布支架是一种满布网孔的管状物,被植入病变的血管以使他们保持开放,药物涂布是为了避免血管发生反复堵塞。
Medtronic shares closed were off about 2 percent, to close at $40.01. .
星期四一项研究结果显示医疗器械公司Medtronic的药物涂布支架与其竞争对手Johnson & Johnson公司同类产品相比具有更高的心脏事件和血栓发生率。其公司股票价格应声下降。
Results from the study, which included more than 2,000 patients, showed that heart patients who received the Medtronic device, called Endeavor, had more heart attacks and blood clots and needed repeat procedures more often than those treated with the Johnson & Johnson stent, known as Cypher.
这项纳入2000多名患者的研究结果显示:植入Medtronic公司的支架- Endeavor-的心脏病患者其心脏事件和血栓发生率以及重复接受手术率要远高于这些应用Johnson & Johnson公司的Cypher的患者。
There was no difference in death rates, however, according to the study, called Sort Out III — which was sponsored by Johnson & Johnson and presented at the Transcatheter Cardiovascular Therapeutics meeting in Washington.
然而,根据这项由Johnson & Johnson公司发起并在华盛顿心血管腔内治疗会议上发布的名为Sort Out III的研究结果显示应用两种产品的死亡率无明显差异。
A Medtronic spokesman, Joe McGrath, said the study was at odds with every other study that has been done.
Medtronic的发言人- Joe McGrath –宣称这项研究与其他各项研究结果不符。
“The data are also short-term,” he said in a statement. “At nine months, it’s premature to draw any meaningful conclusions about a difference between Cypher and Endeavor.”
“这项研究的数据是来自于短期研究”他在一项声明中说“在9个月时间内,希望得出任何关于Cypher和 Endeavor之间差别都是不成熟的。”
“Sort Out III at nine months has no bearing on what matters most to patients with coronary artery disease — long-term freedom from adverse events and long-term freedom from repeat procedures,” he added.
他又说到“Sort Out III在9个月时间内,并没有对冠状动脉及病患者所要达到的重要目标进行研究—长期无副作用和长期免于重复手术”。
In a statement, Dr. Campbell Rogers, chief scientific officer at Johnson & Johnson’s cardiovascular unit, the Cordis Corporation, said, “The breadth of clinical safety and efficacy data and the long-term patient outcomes associated with the Cypher stent are completely unmatched by the competition, and this gap continues to widen.”
Johnson & Johnson 公司心血管部- the Cordis Corporation -的首席科学家Dr. Campbell Rogers在一项声明中说道“Cypher支架与其他支架相比其临床安全性和有效性数据以及患者的长期预后存在差距,而这种差距仍在加大”。
A Leerink Swann analyst, Rick Wise, said he thought the market had overreacted to the data.
Leerink Swann的一位分析员Rick Wise说道他认为市场对这项数据的反应是过激的。
He noted that the study did not directly compare the two devices and that the registries raised questions about, among other things, patient complexity and optimal use of anticlotting drugs, both of which are crucial variables in driving adverse events.
他宣称这项研究并没有对这两种支架直接进行比较。研究者在许多事物中对患者的复杂性和选用适当的涂布药物提出疑问,而这两项是避免副作用的重要参数。
David Lewis, an analyst with Morgan Stanley, characterized the results as a “modest negative” for Medtronic, which already is struggling to defend its market share.
Morgan Stanley的一名分析员David LewisMedtronic认为这项结果对Medtronic来说是一种“适中的负面效应”,而该公司正为维持其股票而挣扎。
Drug-coated stents are tiny wire-mesh tubular devices that are implanted in diseased vessels to prop them open. The drug coating aims at keeping vessels from reclogging.
药物涂布支架是一种满布网孔的管状物,被植入病变的血管以使他们保持开放,药物涂布是为了避免血管发生反复堵塞。
Medtronic shares closed were off about 2 percent, to close at $40.01. .
相关热词搜索:
上一篇:卫生部关于规范新型农村合作医疗健康体检工作的意见
下一篇:民政部推出抗震救灾捐款查询系统
频道总排行
频道本月排行
热门购物
评论排行
- 2011年临床执业医师考试实践技能真...(13)
- 腋臭手术视频(12)
- 2008年考研英语真题及参考答案(5)
- 节食挑食最伤女人的免疫系统(5)
- 核辐射的定义和单位(5)
- CKD患者Tm与IMT相关(5)
- 齐鲁医院普外科开展“喉返神经监护...(5)
- windows7激活工具WIN7 Activation v1.7(5)
- 正常微循环(5)
- 美大学性教育课来真的 男女上阵亲...(4)