从一例手术室内心肺复苏看新版ACLS
2011-09-01 12:05:55 来源: 作者: 评论:0 点击:
這是上個禮拜六值班的病例,男性,38歲,一星期前感冒,於診所施打一劑退燒針(阿司匹林),並發全身皮膚廣泛性脫落丶壞死及黏膜糜爛,入院前兩天解黑便,由外院轉入,診斷,史帝夫強生症候群(Steven Johnson Syndrome)。內視鏡檢查發現瀰漫性消化道黏膜淺潰瘍,於十二指腸有一深度潰瘍,懷疑穿孔,術後轉入內科加護病房,病人抱怨腹痛,腹部CT發現氣腹(peumoperitoneum),遂會診外科,排急診剖腹探查修補術。
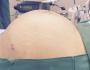
術前評估,患者體壯,體重87kg, 全身皮膚班塊(癒合中),口腔嘴唇眼瞼,有潰爛出血現象,其餘檢查無異常。常規快速誘導,使用fentanyl 2ml, propofol 110mg, succinylcholine 80 mg. ,以(地氟)Desflurane ,Cis-atracurium 維持麻醉。誘導完畢,跟我一起值班的第四年住院醫師,置放動脈導管,當時雖認為這種刀不需要有創動脈壓監測,不過,因為皮膚病灶的關係,使用Cuff-Bp 測量或許有些不便,所以也就沒說什麼!第一次routine的動脈氣體分析,血紅素 11.3 ,沒什麼特別異常之處。
手術約2個小時多一點,失血,手術室報150c.c, 腹血水報250cc.,結束前,住院醫師又抽了一個血氣,血紅素8.6,也與臨床班配,她想輸血,不過外科醫師拒絕。術中血壓最低收縮壓約在100 mmHg, 結束前前也有130 mmHg, 之前有補500 cc 的colloid (6% Hydroxyethylstarch),打完 reverse dose ( vagostigmine + atropine),恢復自主呼吸,收縮壓升到150~160 mmHg ,心跳由80~90升到100-110左右,拔管後,病人醒來,非常躁動,住院醫師在我衝入手術房前已經打入丙泊酚propofol 3ml,此時病人整個人要爬起來,將鼻胃管扯掉,此時心率為130/min, 動脈導管置放在左手腕處,用力掙扎的關係,看不到讀數,病人大聲喊,吸不到氣,這時我又打了3 ml的丙泊酚,結果,心率一下掉到75/min,病人全身軟趴,動脈導管成無波動直線約在 50 mmHg 左右,迅速建立氣道,沒用藥下,插入氣管內管,叫人打epinephrine,開始胸外按摩,壓沒幾下,從口鼻冒出大量血液,鼻胃管插入後,抽出近1000 ml 新鮮紅色血液,此時強烈懷疑上消化道出血,低血容性休克,還好病人原來就有兩條股靜脈(14, 18 gauge),兩條周邊靜脈(20,22 gauge),在血未送到前,給予1000 ml 的代血漿(6% Hydroxyethylstarch),胸外按壓的動脈讀數一直不理想,約在50 mmHg,於是又輸入500 cc 代漿,之後就是迅速取血,按壓,打強心藥,藥物是epinephrine與 Vasopressin,按壓是我與兩個外科住院醫師輪流,至少復甦有60幾分鐘,前2/3幾乎沒有恢復有效的自主循環,後面1/3,間斷性有恢復ROSC,但支撐沒幾分鐘又成為VF,總共電擊三次,epinephrine 使用六次,每次1 mg, Vasopressin 兩次,每次40 U,Atropine前面有打 一次 1mg (住院醫師打的)。 總計最後由鼻胃管流失超過2000 ml, 輸血也超過 10 單位,恢復自主循環後,以epinephrine與dopamine維持血壓,dopamine 用 低劑量, 3 ug/kg.min. Epinephrien 從 1mg/hr 迅速降到 50 ug/hr,30 ug/hr, 出開刀房前停掉。 病人恢復心跳與循環後,自主呼吸恢復,但無其他肢體的動作,亦無意識恢復的現象,住院醫師有看瞳孔大小與反射,瞳孔是放大,對光有微反應。『抱歉,更正,今早再與住院醫師確認,心肺復甦後,兩邊瞳孔不等大,0.1-0.2,與0.3-0.4 mm, 對光有反應,第二次手術結束後,兩邊等大,0.1-.02 mm,對光有反應』
外科醫師不確定出血點,急會內科來做胃腸內視鏡,因為還在active bleeding,只大約看到胃後面十二指腸部不斷地冒血,這期間又費了一個多小時,於是外科再次開下去,加計最後總共失血超過4000 ml.
所有的動脈血氣整理成一個表格,復甦期間是由22:15~23:15分。
[img =http://imageshack.us/img30/8310/94179849.pngimg30./][/IMG]
討論:
1. 復甦過程的用藥是否符合新版ACLS的規範。
2. 新版ACLS強調高品質胸外按壓的意義與實踐。
3.ACLS 終止的判定。
4.Sodium bicarbonate 碳酸氫鈉使用與否與時機。
5. 鈣劑的使用與否。
6. Amiodarone 的使用與否。
7. 復甦後(ROSC),是否採用低溫療法的判斷。
8. 休克後與大量輸血後併發症的監測。
心肺复苏后由于使用了大量的肾上腺素,因为倍他受体激动,瞳孔是增大的,且对光反射迟钝,因此应在倍他受体激动解除后,再通过瞳孔评价脑功能。
倍他受体激动还有另外一个作用就是使细胞外的K离子进入细胞内,因此我们在心肺复苏后复查血气,虽有严重代酸,但K离子并不明显增高。
"史帝文生強生症候群"简介:
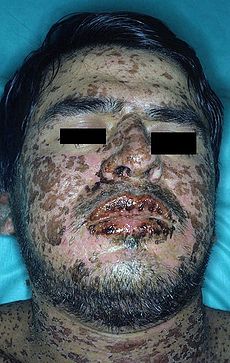
它是一種致命的皮膚藥物過敏反應,病患因服用藥物或病毒感染後會發燒、全身皮膚、黏膜起水泡及潰爛,症狀嚴重者全身有如燙傷,又稱「毒性表皮溶解症」。
史帝文生強生症候群的致命基因已被台湾研究人員在2004年找出.其表现为有些人吃藥後可引發全身皮膚黏膜潰爛、器官衰竭。
史帝文生強生症候群的致病機轉不明,且沒有良好的治療藥物,只能依賴全身類固醇治療。因此,死亡率偏高,如果存活,高達一成患者會失明,或是出現視力減退,甚至腎衰竭等後遺症。
繼2004年台灣研究團隊領先全球找到了嚴重皮膚藥物過敏反應「史帝文生強生症候群」的致病基因,成果刊登於「自然」期刊後,研究團隊又找到了此病症最終的致命毒性蛋白與治療此症的治療標的。成果刊登於「自然醫學」期刊,獲得國際學術界肯定。
該研究發現了,「史帝文生強生症候群」的免疫機轉,由於毒殺T細胞或自然殺手細胞的極度失控,釋放出大量細胞毒性蛋白--「顆粒溶解素」。毒性蛋白在病患皮膚黏膜大量釋放堆積,啟動皮膚表皮的細胞凋亡路徑,最後引起大量表皮細胞壞死。
在正常人體內,「顆粒溶解素」可說是重要的生物防護系統,具有毒殺外來病菌或惡性腫瘤細胞的超強能力。特殊體質病患服用特定藥物時,這類毒性蛋白分不清敵我,擴散到全身,造成皮膚、黏膜細胞的死亡,甚至引起器官衰竭。
此研究解開嚴重皮膚藥物過敏反應毒殺細胞自體免疫反應傷害人體的重要機轉,也為此病症找到關鍵的治療標的,未來針對此毒性蛋白可開發中合性抗體或抑制性藥物,大幅減低死亡率及後遺症。
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN)[1] are two forms of a life-threatening skin condition, in which cell death causes the epidermis to separate from the dermis. The syndrome is thought to be a hypersensitivity complex that affects the skin and the mucous membranes. Although the majority of cases are idiopathic (without a known cause), the main class of known causes is medication, followed by infections and, rarely, cancers.
| Stevens–Johnson syndrome |
| Classification and external resources |
 |
Classification
There is agreement in the medical literature that Stevens–Johnson syndrome (SJS) can be considered a milder form of toxic epidermal necrolysis (TEN). These conditions were first recognised in 1922.[2]
Both diseases can be mistaken for erythema multiforme. Erythema multiforme is sometimes caused by a reaction to a medication but is more often a type III hypersensitivity reaction to an infection (caused most often by Herpes simplex) and is relatively benign. Although both SJS and TEN can also be caused by infections, they are most often adverse effects of medications. Their consequences are potentially more dangerous than those of erythema multiforme.
Signs and symptoms
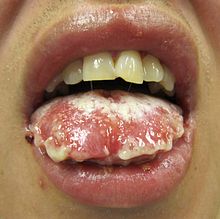
![]() Mucosal desquamation in a person with Steven Johnson's syndrome
Mucosal desquamation in a person with Steven Johnson's syndrome
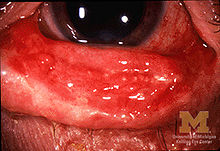
![]() Conjunctivitis (inflammation of eye and eyelid) in SJS
Conjunctivitis (inflammation of eye and eyelid) in SJS
SJS usually begins with fever, sore throat, and fatigue, which is misdiagnosed and usually treated with antibiotics. Ulcers and other lesions begin to appear in the mucous membranes, almost always in the mouth and lips but also in the genital and anal regions. Those in the mouth are usually extremely painful and reduce the patient's ability to eat or drink. Conjunctivitis of the eyes occurs in about 30% of children who develop SJS. A rash of round lesions about an inch across arises on the face, trunk, arms and legs, and soles of the feet, but usually not the scalp.[3]
Causes
SJS is thought to arise from a disorder of the immune system.[3]
Infections
It can be caused by infections (usually following infections such as herpes simplex virus, influenza, mumps, cat-scratch fever, histoplasmosis, Epstein-Barr virus, mycoplasma pneumoniae or similar).
Medication/drugs
See also: List of SJS inducing substances
It can be caused by adverse effects of drugs (allopurinol a.k.a. Aloprim, Zyloprim, Dilantin, Depakote, Levaquin, diclofenac, etravirine, isotretinoin a.k.a. Accutane, fluconazole,[4] valdecoxib, sitagliptin, oseltamivir, penicillins, barbiturates, sulfonamides, phenytoin, azithromycin, oxcarbazepine, zonisamide, modafinil,[5] lamotrigine, nevirapine, pyrimethamine, ibuprofen,[6] ethosuximide, carbamazepine, nystatin, and gout medications).[7][8]
Although Stevens–Johnson Syndrome can be caused by viral infections, malignancies or severe allergic reactions to medication, the leading cause appears to be the use of antibiotics and sulfa drugs.
Medications that have traditionally been known to lead to SJS, erythema multiforme and toxic epidermal necrolysis include sulfonamides (antibiotics), penicillins (antibiotics), barbiturates (sedatives), lamotrigine and phenytoin (e.g. Dilantin) (anticonvulsants). Combining lamotrigine with sodium valproate increases the risk of SJS.
Non-steroidal anti-inflammatory drugs are a rare cause of SJS in adults; the risk is higher for older patients, women and those initiating treatment.[2] Typically, the symptoms of drug-induced SJS arise within a week of starting the medication. People with systemic lupus erythematosus or HIV infections are more susceptible to drug-induced SJS.[3]
SJS may also be caused by cocaine usage.[9]
[edit] Genetics
In some East Asian populations studied (Han Chinese and Thai), carbamazepine- and phenytoin-induced SJS is strongly associated with HLA-B*1502 (HLA-B75), an HLA-B serotype of the broader serotype HLA-B15.[10][11][12] A study in Europe suggested that the gene marker is only relevant for East Asians.[13][14] Based on the Asian findings, similar studies in Europe showed 61% of allopurinol-induced SJS/TEN patients carried the HLA-B58 (B*5801 allele - phenotype frequency in Europeans is typically 3%). One study concluded, "Even when HLA-B alleles behave as strong risk factors, as for allopurinol, they are neither sufficient nor necessary to explain the disease."[15]
Treatment
SJS constitutes a dermatological emergency. All medications should be discontinued, particularly those known to cause SJS reactions. Patients with documented mycoplasma infections can be treated with oral macrolide or oral doxycycline.[3]
Initially, treatment is similar to that for patients with thermal burns, and continued care can only be supportive (e.g. intravenous fluids and nasogastric or parenteral feeding) and symptomatic (e.g., analgesic mouth rinse for mouth ulcer). Dermatologists and surgeons tend to disagree about whether the skin should be debrided.[3]
Beyond this kind of supportive care, there is no accepted treatment for SJS. Treatment with corticosteroids is controversial. Early retrospective studies suggested that corticosteroids increased hospital stays and complication rates. There are no randomized trials of corticosteroids for SJS, and it can be managed successfully without them.[3]
Other agents have been used, including cyclophosphamide and cyclosporine, but none has exhibited much therapeutic success. Intravenous immunoglobulin (IVIG) treatment has shown some promise in reducing the length of the reaction and improving symptoms. Other common supportive measures include the use of topical pain anesthetics and antiseptics, maintaining a warm environment, and intravenous analgesics. An ophthalmologist should be consulted immediately, as SJS frequently causes the formation of scar tissue inside the eyelids, leading to corneal vascularization, impaired vision and a host of other ocular problems.
Prognosis
SJS proper (with less than 10% of body surface area involved) has a mortality rate of around 5%. The risk for death can be estimated using the SCORTEN scale, which takes a number of prognostic indicators into account.[9] Other outcomes include organ damage/failure, cornea scratching and blindness.
[edit] Epidemiology
Stevens–Johnson syndrome is a rare condition, with a reported incidence of around 2.6[3] to 6.1[2] cases per million people per year. In the United States, there are about 300 new diagnoses per year. The condition is more common in adults than in children. Women are affected more often than men, with cases occurring at a two to one (2:1) ratio.[2]
History
Stevens-Johnson Syndrome is named for Albert Mason Stevens and Frank Chambliss Johnson, American pediatricians who in 1922 jointly published a description of the disorder in the American Journal of Diseases of Children.[16][17][18][19]
Notable cases
- Padma Lakshmi, actress, model, television personality, and cookbook writer;[20]
- Tessa Keller of MTV show Laguna Beach;[21]
- Sabrina Brierton Johnson, whose family unsuccessfully sued the manufacturer of Children's Motrin, Johnson & Johnson, after a case of SJS blinded her.[22]
- Manute Bol, former professional basketball player and member of NBA's Washington Bullets, Golden State Warriors, Philadelphia 76ers, and Miami Heat, who died from complications.[23]
频道总排行
频道本月排行
热门购物
评论排行
- 2011年临床执业医师考试实践技能真...(13)
- 腋臭手术视频(12)
- 2008年考研英语真题及参考答案(5)
- 节食挑食最伤女人的免疫系统(5)
- 核辐射的定义和单位(5)
- CKD患者Tm与IMT相关(5)
- 齐鲁医院普外科开展“喉返神经监护...(5)
- windows7激活工具WIN7 Activation v1.7(5)
- 正常微循环(5)
- 美大学性教育课来真的 男女上阵亲...(4)